HEBEI GEMMY IMP.& EXP.CO.,LTD , https://www.gemmycandles.com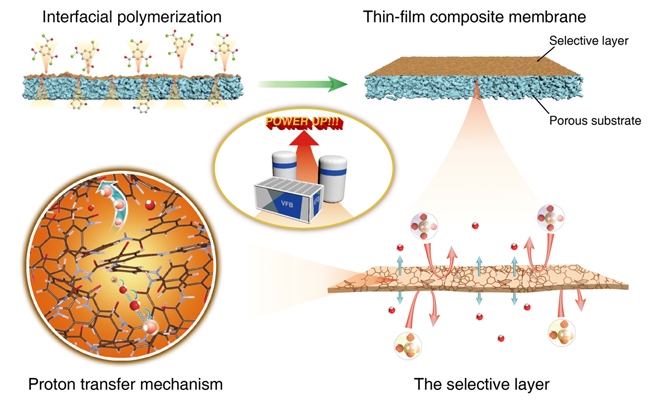
High selective permeability separation layer composite ion conductive membrane improves the performance of flow battery
[ Instrument Network Instrument R & D ] Recently, Li Xianfeng and Zhang Huamin, researchers of the Energy Storage Technology Research Department (DNL17) of the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, have developed a high selective permeability ultra-thin separation layer composite ion-conducting membrane. Ionic conductivity and high ion selectivity can greatly improve the performance of flow batteries.
The electrochemical flow battery is generally called a redox flow battery, which is a new type of large-scale electrochemical energy storage device. The positive and negative electrodes are all-vanadium flow batteries, which are called vanadium batteries. At 100% state, the open circuit voltage of the battery can reach 1.5 V.
Flow battery is a new type of storage battery. The flow battery is a high-performance battery separated by positive and negative electrolytes and circulated separately. It has the characteristics of high capacity, wide application area (environment), and long cycle life. New energy products. The redox flow battery is a new type of large-capacity electrochemical energy storage device that is being actively researched and developed. It is different from the battery that usually uses solid material electrodes or gas electrodes. Its active material is a flowing electrolyte solution. Its characteristics are scale Under the rising demand for the widespread use of renewable energy, it is foreseeable that the flow battery will usher in a period of rapid development.
Ion-conducting membrane material is the key material of flow battery. Its role is to block active materials at both ends and transfer carriers to form a battery circuit. The team broke through the traditional "ion exchange transfer" mechanism in the early stage, and originally proposed the concept of "ion screening conduction" without ion exchange groups (Energy. Environ. Sci., 2011, 4, 1676), which will be porous An ion-conducting membrane is introduced into the flow battery. Based on this, a lot of research work has been carried out on the structural design of high-performance porous ion-conducting membranes, and a series of progress have been made.
How to break the ion-selective and ion-conducting Trade-Off effect of the membrane and develop an ion-conducting membrane material with both high selectivity and high conductivity is a difficult research point in the field of porous ion-conducting membranes for flow batteries. Porous ion-conducting membrane materials prepared by traditional phase inversion generally have an asymmetric structure, high pore curvature, poor penetration, and low ion conductivity. In contrast, the composite membrane has a selective separation layer and support layer structure that can be individually controlled, which is expected to break through the Trade-Off effect between membrane selectivity and conductivity and further improve the performance of flow batteries.
Polyamide is commonly known as nylon (Nylon), English name Polyamide, it is a general term for polymers containing amide groups in the repeating unit of the main chain of the macromolecule. Polyamides can be obtained by ring-opening polymerization of lactams, or by polycondensation of diamines and diacids. Polyamide (PA) refers to a polymer containing a polar amide group (-CO-NH-) in the main chain. Originally used as a raw material for manufacturing fibers, PA has become a widely used engineering plastic in the industry due to its toughness, wear resistance, self-lubrication, and wide temperature range. PA is widely used to replace copper, non-ferrous metal making machinery, chemical, and electrical parts, such as diesel engine fuel pump gears, water pumps, high-pressure seals, oil pipelines, etc.
In this work, an interfacial polymerization technique was used to prepare a separation membrane with an ultra-thin separation layer. The separation membrane consists of a polyamide crosslinked network with a thickness of only 180 nm. This thickness greatly reduces the ion transmission path and improves membrane selectivity. At the same time, the free volume inside the polyamide cross-linked network is between hydrated protons and hydrated vanadium ions, which can effectively block vanadium ions and transfer protons at the same time, so that the membrane has high ion selectivity. The single cell assembled with this membrane material has an energy efficiency of more than 80% at a current density of 260 mA / cm2. In addition, the team collaborated with Zheng Anmin, a researcher at the Wuhan Institute of Physics and Mathematics of the Chinese Academy of Sciences, to study the proton transfer mode in the polyamide separation layer through theoretical calculations. The results show that the protons can pass through the water molecular chain and polyamide skeleton in the polyamide network. The carboxyl group on the jump is passed by the Grottuss mechanism. This research result provides new ideas for designing high-performance ion-conducting membranes.